Wolfram Function Repository
Instant-use add-on functions for the Wolfram Language
Function Repository Resource:
Estimate physical properties of chemicals using Joback fragmentation
ResourceFunction["JobackEstimate"][molecule,property] gives an estimate of the specified property for the given molecule using Joback fragmentation. |
| "CriticalPressure" | critical pressure (bar) |
| "CriticalTemperature" | critical temperature (K) |
| "CriticalVolume" | critical volume (mL/mol) |
| "EnthalpyOfFormation" | enthalpy of formation (kJ/mol) |
| "EnthalpyOfFusion" | enthalpy of fusion (kJ/mol) |
| "EnthalpyOfVaporization" | enthalpy of vaporization (kJ/mol) |
| "GibbsEnergyOfFormation" | Gibbs energy of formation (kJ/mol) |
| {"IdealGasHeatCapacity",t} | ideal gas heat capacity at temperature t (J/(mol·K)) |
| {"LiquidViscosity",t} | liquid viscosity at temperature t (Pa·s) |
| "NormalBoilingPoint" | normal boiling point (K) |
| "NormalFreezingPoint" | normal freezing point (K) |
Estimate the boiling point of acetone:
| In[1]:= |
| Out[1]= |
Compare with the known boiling point:
| In[2]:= |
| Out[2]= |
A molecule:
| In[3]:= |
| Out[3]= |
Estimate a single property:
| In[4]:= |
| Out[4]= |
Estimate multiple properties:
| In[5]:= |
| Out[5]= |
A molecule:
| In[6]:= |
| Out[6]= |
Plot its ideal gas heat capacity and liquid viscosity over a given temperature range:
| In[7]:= | ![{Plot[ResourceFunction["JobackEstimate"][
mol, {"IdealGasHeatCapacity", Quantity[T, "Kelvins"]}], {T, 273., 1000.}, {AxesLabel -> {"T",
Subsuperscript["C", "p", 0]}, PlotLabel -> "IdealGasHeatCapacity"}], Plot[ResourceFunction["JobackEstimate"][
mol, {"LiquidViscosity", Quantity[T, "Kelvins"]}], {T, 287.3, 550.3}, {AxesLabel -> {"T",
Subscript["\[Eta]", "L"]}, PlotLabel -> "LiquidViscosity"}]} // GraphicsRow](https://www.wolframcloud.com/obj/resourcesystem/images/fca/fca3dcc9-e1d3-4f4c-9f38-29b6e9fe8a64/12a0049b50e2f375.png) |
| Out[7]= | 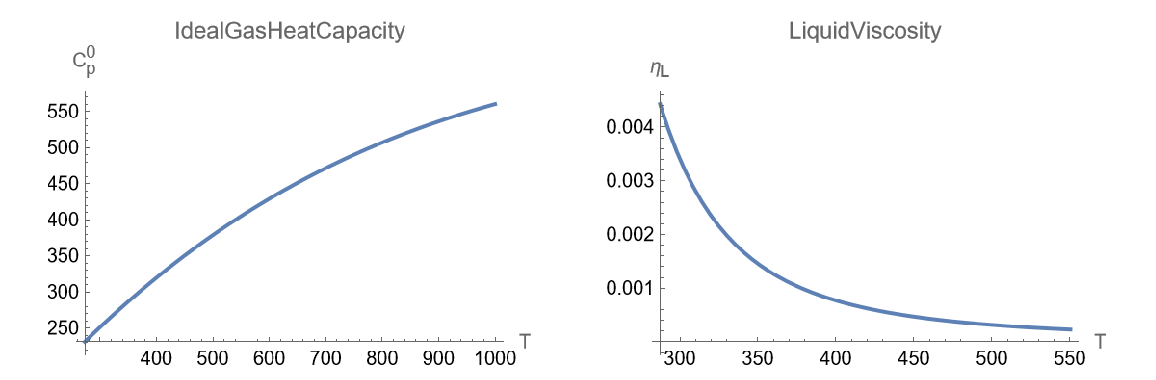 |
Molecules that contain functional groups not supported by the Joback method will return Missing:
| In[8]:= |
| Out[8]= |
Wolfram Language 12.3 (May 2021) or above
This work is licensed under a Creative Commons Attribution 4.0 International License