Wolfram Function Repository
Instant-use add-on functions for the Wolfram Language
Function Repository Resource:
Neutralize the ionic form of a molecule provided as a Molecule, SMILES or InChI string
ResourceFunction["MoleculeNeutralize"][m] neutralizes the charges on a molecule m specified as a Molecule, SMILES string or InChI string. | |
ResourceFunction["MoleculeNeutralize"][m,format] changes the output format to differ from the input format. | |
ResourceFunction["MoleculeNeutralize"][m,format,options] specifies additional or substitute rules for the neutralization reactions as options. |
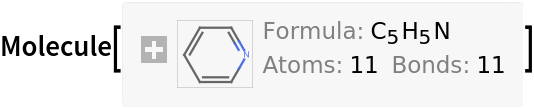
Inputs provided as Molecule are returned as Molecule:
| In[1]:= | ![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/acfda4ed-c427-4d7b-bf9a-a08a49dc5808"]](https://www.wolframcloud.com/obj/resourcesystem/images/d22/d22946dc-a71b-4dcc-8cb1-7d616520d644/4f60ed0feb757696.png) |
Inputs provided as a SMILES strings are returned as SMILES strings:
| In[2]:= |
| Out[2]= |
Inputs provides as InChI strings are returned as InChI strings:
| In[3]:= |
| Out[4]= |
Lists of inputs can be provided, and each element is processed according to its appropriate type:
| In[5]:= | ![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/edf8910d-a553-4214-9aac-73b22913799d"]](https://www.wolframcloud.com/obj/resourcesystem/images/d22/d22946dc-a71b-4dcc-8cb1-7d616520d644/2fb7fbb880c5e3b5.png) |
| Out[5]= |
All possible neutralizations are performed if a molecule contains multiple charged sites:
| In[6]:= |
| Out[6]= |
Only neutralization reactions that can be achieved by adding or removing protons without breaking other bonds are performed; as a result, the final molecule may not have charge 0. For example, a quaternary ammonium must have a positive charge to satisfy valence rules:
| In[7]:= |
| Out[7]= |
By default, MoleculeNeutralize returns the molecule in the same representation it was provided. Setting the second argument overrides this; choices include "InChI", "InChIKey", "Molecule" and "SMILES":
| In[8]:= |
| Out[8]= |
| In[9]:= |
| Out[9]= |
| In[10]:= |
| Out[10]= |
| In[11]:= |
| Out[11]= |
The "UseRules" option allows you to specify a list of reaction rules that will be used instead of the default charge neutralization rules:
| In[12]:= |
| Out[12]= |
The "AddRules" option allows you to specify a list of reaction rules that will be applied after the default charge neutralization rules have been applied:
| In[13]:= |
| Out[13]= |
This work is licensed under a Creative Commons Attribution 4.0 International License