Basic Examples (3)
Define a chemical reaction:
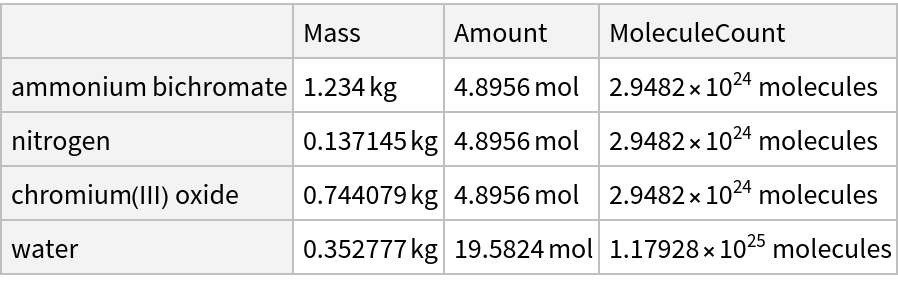
Summarize the products and reactants given 1.234 kg of ammonium bichromate:
Get the result as an Association:
Applications (8)
Specify the reactants as chemical entities:
Specify the chemical formulas as strings:
We can also use Molecule:
Get the reaction data using the entity-based reaction:
The keys are entities:
Get the reaction data using the formula-based reaction:
The keys are ChemicalFormula objects:
Get the reaction data using the molecule-based reaction:
The keys are Molecule objects:
There's one more way to do it—by just entering the reaction as a string. This might not work when you have isomers, though:
Synthesis Reactions (4)
One type is a reaction of an element with oxygen and sulfur. Get the reaction data for a random mass of the reactant:
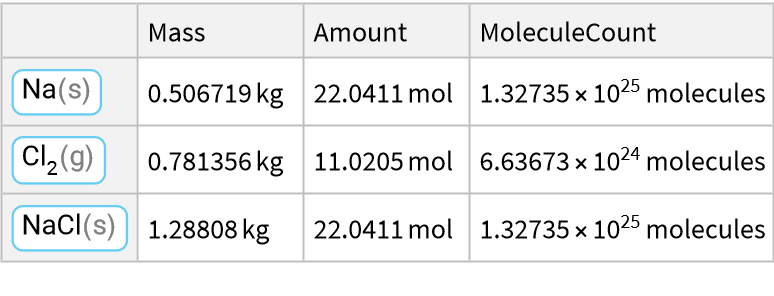
Here is a reaction of a metal with a halogen:
There's also the option to specify a different product or reactant:
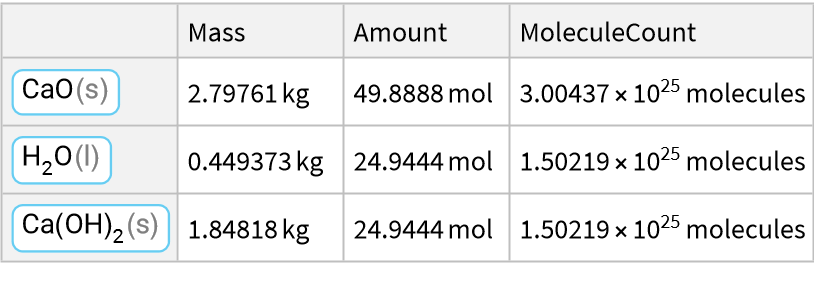
Synthesis reaction with oxides:
Decomposition Reactions (1)
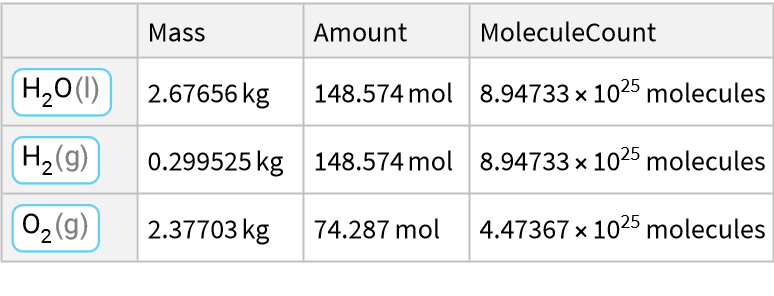
Water decomposes into hydrogen gas and oxygen gas:
Single Displacement Reactions (1)
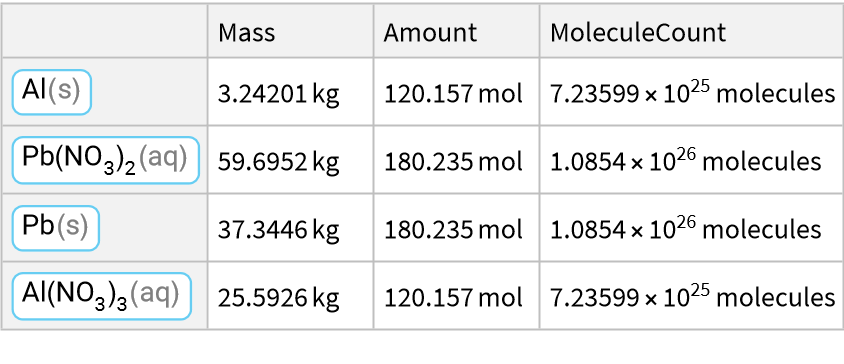
This is a single displacement reaction/single replacement reaction: Aluminum reacts with plumbous nitrate/lead (II) nitrate to produce lead and aluminium nitrate:
Double Displacement Reactions (1)
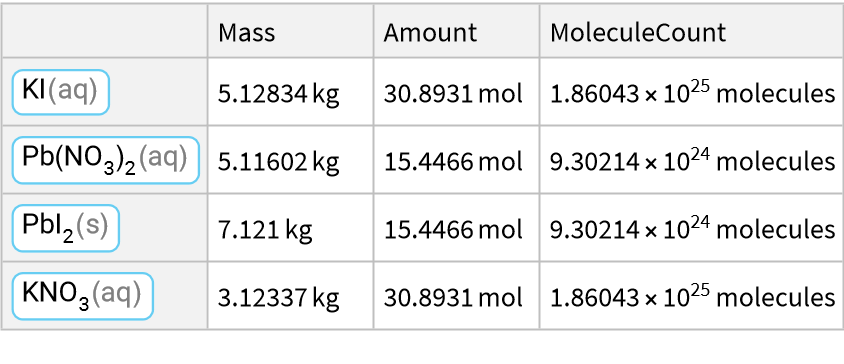
This is a double displacement reaction/double replacement reaction/metathesis reaction: Potassium iodide reacts with lead(II) nitrate to produce lead(II) iodide and potassium nitrate/saltpeter:
Combustion Reactions (1)
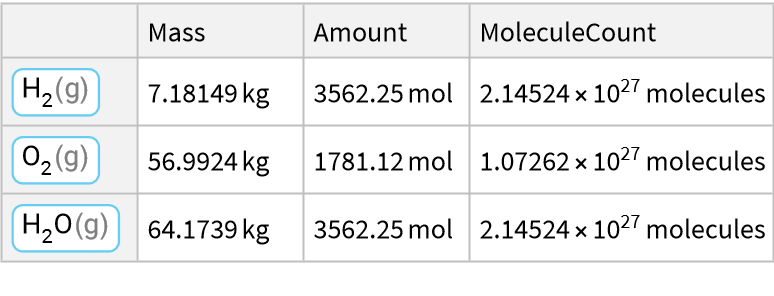
A combustion reaction for hydrogen gas:
![reaction = ChemicalReaction[<|
Entity["Chemical", "AmmoniumDichromate"] -> 1|> -> <|
Entity["Chemical", "MolecularNitrogen"] -> 1, Entity["Chemical", "ChromiumIIIOxide"] -> 1, Entity["Chemical", "Water"] -> 4|>]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/038e98dc819df335.png)
![ResourceFunction["ReactionData"][reaction, ChemicalInstance[Entity["Chemical", "AmmoniumDichromate"], Quantity[1.234`, "Kilograms"]], "Association"]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/28dff248f369a096.png)

![entityform = ChemicalReaction[<|
Entity["Chemical", "AmmoniumDichromate"] -> 1|> -> <|
Entity["Chemical", "MolecularNitrogen"] -> 1, Entity["Chemical", "ChromiumIIIOxide"] -> 1, Entity["Chemical", "Water"] -> 4|>]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/6aeb43c085bd5624.png)
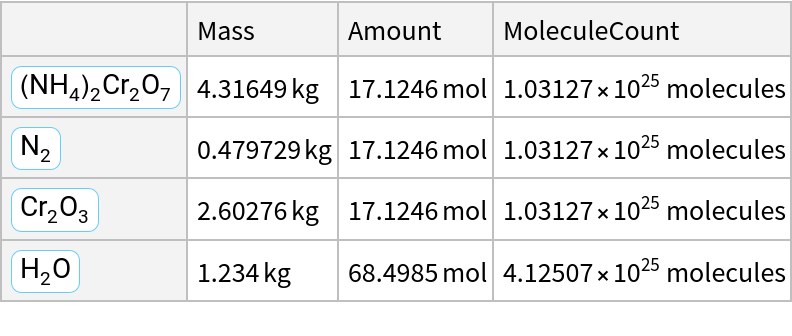
![formulaform = ChemicalReaction[<|ChemicalFormula["(NH4)2Cr2O7"] -> 1|> -> <|
ChemicalFormula["N2"] -> 1, ChemicalFormula["Cr2O3"] -> 1, ChemicalFormula["H2O"] -> 4|>]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/008851f2c29e2534.png)
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/bab9b308-d744-4216-a036-719d7ed21177"]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/6ad70cd3c16b253e.png)

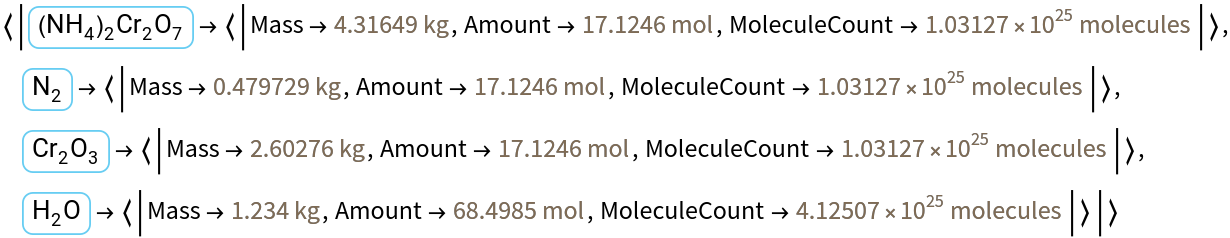
![ResourceFunction["ReactionData"][moleculeform, ChemicalInstance[Molecule[{"O"}, {}, {}], Quantity[1.234`, "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/42e177475a4c8b1d.png)
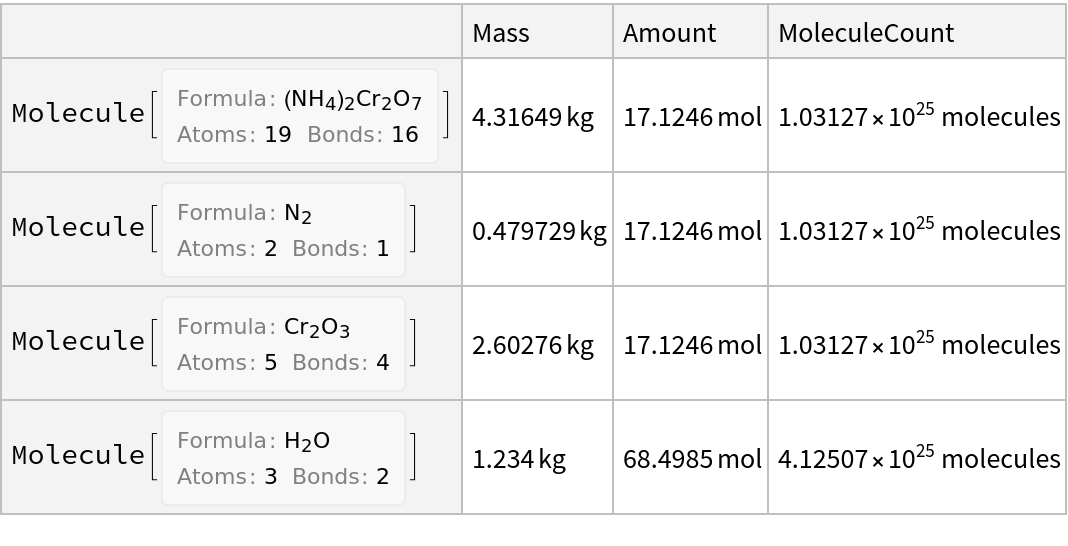
![ResourceFunction["ReactionData"][moleculeform, ChemicalInstance[Molecule[{"O"}, {}, {}], Quantity[1.234`, "Kilograms"]], "Association"]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/4b3a12f8ead7cba4.png)

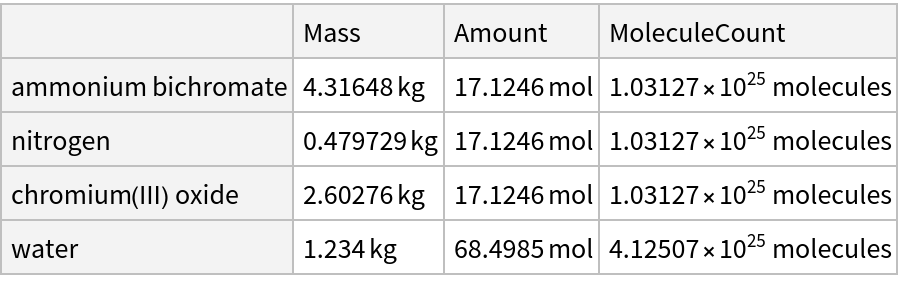
![ResourceFunction["ReactionData"][
ChemicalReaction["(NH4)2Cr2O7 -> N2 + Cr2O3 + H2O"], ChemicalInstance[ChemicalFormula["H2O"], Quantity[1.234`, "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/3542d909b1615dcc.png)

![ResourceFunction["ReactionData"][
ChemicalReaction["2Mg(s)+O2(g)->2MgO(s)"], ChemicalInstance[ChemicalFormula["Mg(s)"], Echo@Quantity[RandomReal[{1, E^2}], "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/0ec2174dcab95dcb.png)
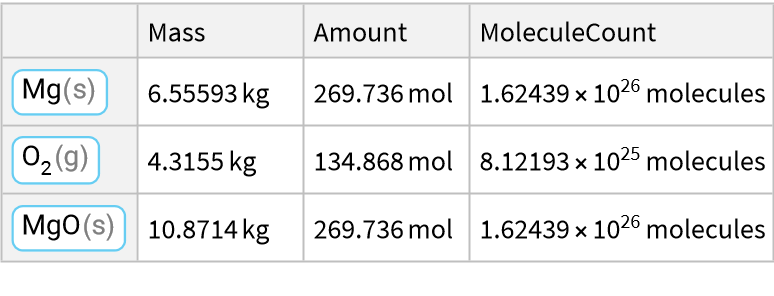
![ResourceFunction["ReactionData"][
ChemicalReaction["2Na(s)+Cl2(g)->2NaCl(s)"], ChemicalInstance[ChemicalFormula["Na(s)"], Echo@Quantity[RandomReal[{1, E^2}], "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/7c720747b03fd5c3.png)
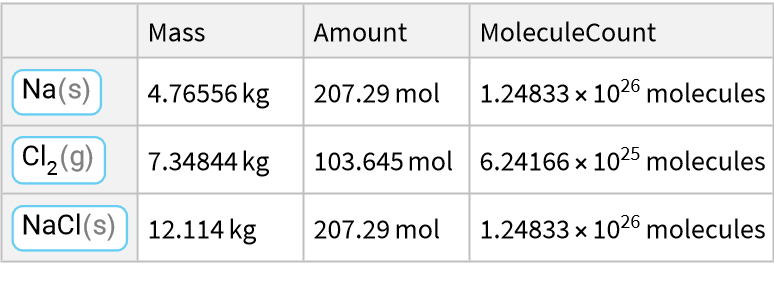
![ResourceFunction["ReactionData"][
ChemicalReaction["2Na(s)+Cl2(g)->2NaCl(s)"], ChemicalInstance[ChemicalFormula["Cl2(g)"], Echo@Quantity[RandomReal[{1, E^2}], "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/023da1a6c27ba324.png)
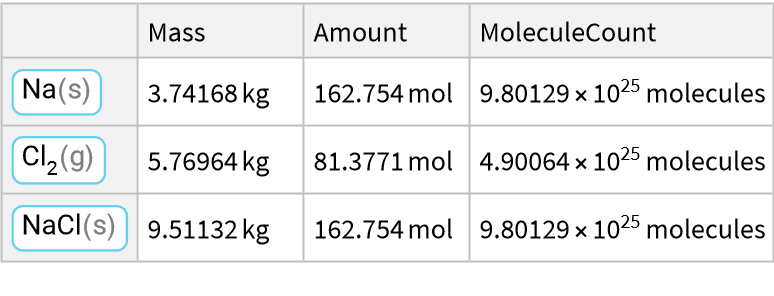
![ResourceFunction["ReactionData"][
ChemicalReaction["2Na(s)+Cl2(g)->2NaCl(s)"], ChemicalInstance[ChemicalFormula["NaCl(s)"], Echo@Quantity[RandomReal[{1, E^2}], "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/0521d6f947a42948.png)
![ResourceFunction["ReactionData"][
ChemicalReaction["2CaO(s)+H2O(l)->Ca(OH)2(s)"], ChemicalInstance[ChemicalFormula["CaO(s)"], Echo@Quantity[RandomReal[{1, E^2}], "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/3642310b00290aa8.png)
![ResourceFunction["ReactionData"][
ChemicalReaction["2H2O(l)->2H2(g)+O2(g)"], ChemicalInstance[ChemicalFormula["H2O(l)"], Echo@Quantity[RandomReal[{1, E^2}], "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/29d327447e671bbe.png)
![ResourceFunction["ReactionData"][
ChemicalReaction["2Al(s)+3Pb(NO3)2(aq)->3Pb(s)+2Al(NO3)3(aq)"], ChemicalInstance[ChemicalFormula["Al(s)"], Echo@Quantity[RandomReal[{1, E^2}], "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/630a5dc7f211bbf3.png)
![ResourceFunction["ReactionData"][
ChemicalReaction["2KI(aq)+Pb(NO3)2(aq)->PbI2(s)+2KNO3(aq)"], ChemicalInstance[ChemicalFormula["PbI2(s)"], Echo@Quantity[RandomReal[{1, E^2}], "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/0a1c21c1024e1c5a.png)
![ResourceFunction["ReactionData"][
ChemicalReaction["2H2(g)+O2(g)->2H2O(g)"], ChemicalInstance[ChemicalFormula["H2(g)"], Echo@Quantity[RandomReal[{1, E^2}], "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/248ae46af9ff4fff.png)
![ResourceFunction[
"ReactionData"][ChemicalReaction[<|Entity[
"Chemical", "AmmoniumDichromate"] -> 1|> -> <|Entity[
"Chemical", "MolecularNitrogen"] -> 1, Entity["Chemical", "ChromiumIIIOxide"] -> 1, Entity["Chemical", "Water"] -> 4|>], ChemicalInstance[Entity["Chemical", "SodiumChloride"], Quantity[1.234`, "Kilograms"]]]](https://www.wolframcloud.com/obj/resourcesystem/images/cc1/cc14ae3c-c3a6-4520-bbd1-a76f95e27d9d/0da949c32e3770f7.png)