Details and Options
Conversions among various units of density are combined here. Some conversions are not perfect. For example, specific gravity and °Brix (°Bx) do not measure the same physical property, and are often measured using different instruments. Some of these conversions are therefore based on expressions derived from polynomial fits to experimental datasets.
The following units are supported:
| "ApparentSpecificGravity" | apparent specific gravity, used as an IndependentUnit |
| "DegreesBalling" | degrees Balling |
| "DegreesBrix" | degrees Brix |
| "DegreesBabo" | degrees Babo, also know as degrees Klosterneuburger Mostwaage |
| "DegreesBaume" | degrees Baumé |
| "DegreesPlato" | degrees Plato |
| "OechsleDegrees" | Oechsle degrees, short for Oechsle Mostwaage scale |
| "GravityPoints" | gravity points |
| "PotentialAlcohol" | potential alcohol, used as an IndependentUnit, value in %v/v |
| "RefractiveIndex" | refractive index, used as an IndependentUnit |
| "Grams"/"Liter" | grams per liter |
| "GramsOfDissolvedSolidsPerLiter" | grams of dissolved solids per liter |
Apparent specific gravity is the ratio of the weight of a volume of the substance to the weight of an equal volume of the reference substance, here water at its densest (4 °C) and at a pressure of 1 atm. The lower limit is therefore set to 1.
The Balling scale (°Bg, °Blg) was designed especially for measuring dissolved-solids concentrations (mainly sucrose) as a sucrose mass fraction (percentage by weight)—originally at 17.5 °C. It was created in 1843. The Balling scale appears on older saccharimeters and it is still used in the wine industry in South Africa. Here the gravity points method is used.
Degrees Brix (°Bx) are defined by an international standard (ICUMSA). The system was designed to determine how many grams of sucrose are dissolved in 100 g of a sugar aqueous solution. It represents the sugar mass fraction. A measurement of 25 °Bx means that in 100 g of aqueous solution, 25 g of sucrose are dissolved. Sometimes the percent sign (%) is used as a unit also, because the sucrose content is a sucrose percentage by weight. The Brix scale is primarily used for measuring fruit juices and grapes, as well as in the sugar industry.
The Babo scale (°Babo) or Klosterneuburger Mostwaage scale (°KMW, °Kl) indicates how many kilograms of sugar contain 100 kilograms of grape must. It is based on the Balling scale, but it also considers the influence of non-sugars (insoluble solid parts in the fruit juice). It was created in 1891 and used widely in the former Austro-Hungarian Empire.
The Baumé scale (°Baume) was developed as a scale for measuring liquid density. It was created in 1768 and is in use in French-speaking countries and Spain.
The Plato scale (°P) is a recalculation of the Balling scale. It uses a reference temperature of 17.5 °C. It is used primarily in the brewing industry. The Lincoln equation is used for conversion.
The Oechsle scale (Oechsle degrees), also know as Oechsle Mostwaage, is designed for measuring wine-must (grape juice, grape must) sweetness. The value of wine-must sweetness is derived from the wine-must density at 20 °C. It was created around 1820 and is used mainly in Germany, Switzerland and Luxembourg.
The number behind the decimal point of the specific gravity is often referred to as gravity points.
Potential alcohol is not a measure of density. However, based on the sugar content of an aqueous solution, one can estimate the potential alcohol content (%v/v) that can be reached through fermentation. Note that not all the sugar is converted into alcohol—rather, a 90% efficiency is assumed.
Dissolved sugar is not a measure of density. It is an estimate of dissolved solids, assuming that most of the solids are sucrose. It will be close to the true value.
Refractometers are a common way to estimate the sugar content in an aqueous solution via refractive index.
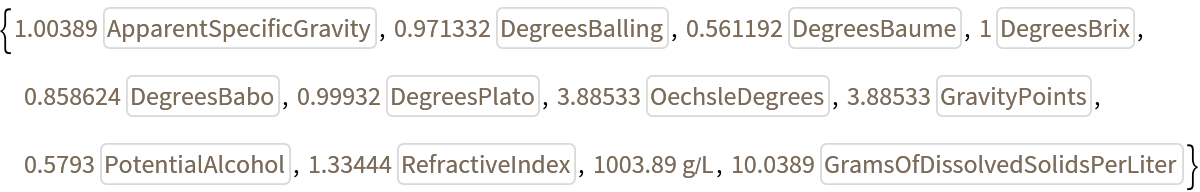
![ResourceFunction["SolutionSugarContentConvert"][
Quantity[1, IndependentUnit["DegreesBrix"]], #] & /@ (Join[
IndependentUnit /@ {"ApparentSpecificGravity", "DegreesBalling", "DegreesBaume", "DegreesBrix", "DegreesBabo", "DegreesPlato", "OechsleDegrees", "GravityPoints", "PotentialAlcohol", "RefractiveIndex"}, {"Grams"/"Liters"}, IndependentUnit /@ {"GramsOfDissolvedSolidsPerLiter"}])](https://www.wolframcloud.com/obj/resourcesystem/images/929/929a5496-20a3-4284-ad0f-483aae842b9d/704a69967b68a563.png)