Basic Examples (12)
Identify a"cis" square-planar molecule, with same-element atoms next to each other::
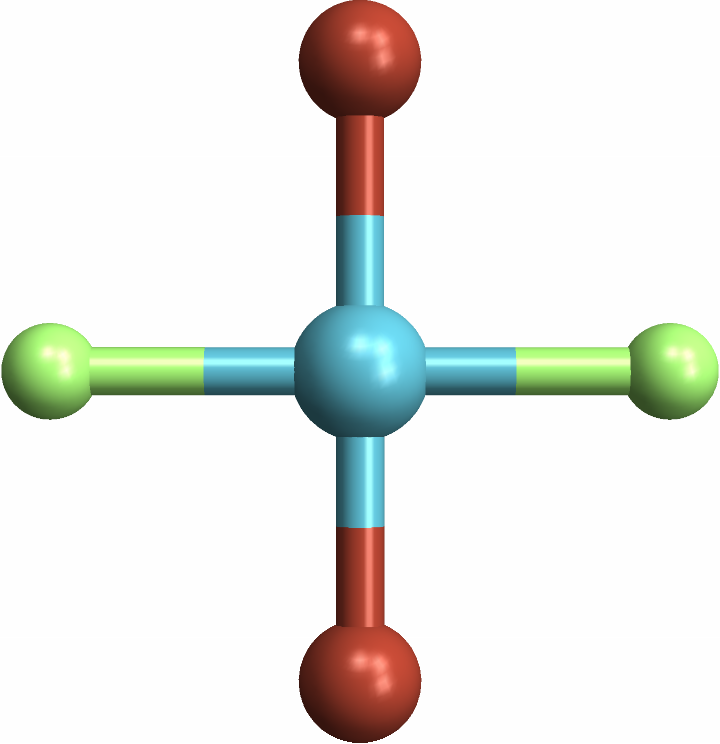
Identifying "trans" square-planar molecules, with same-element atoms across from each other:
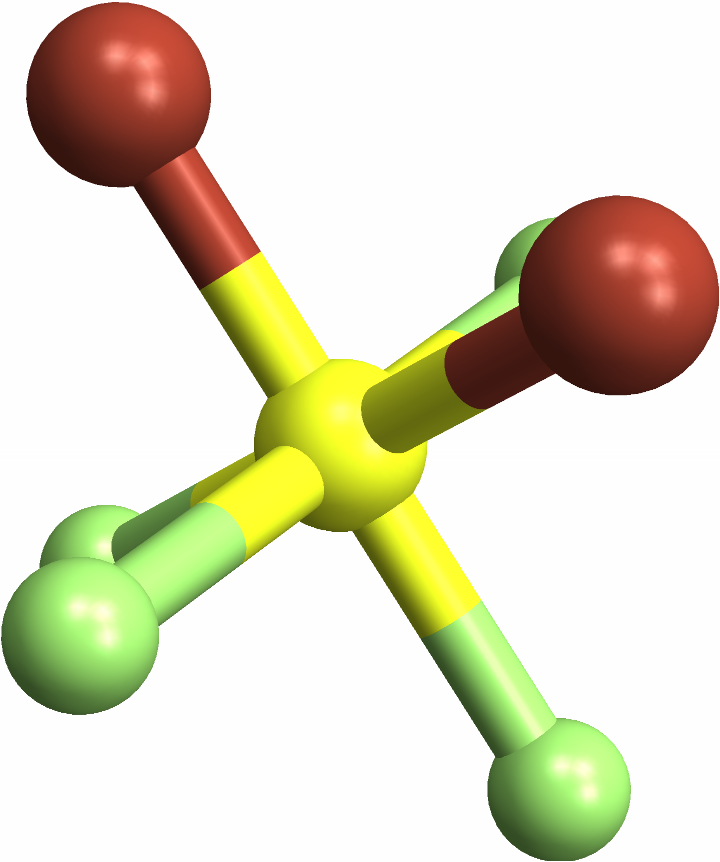
Identifying "cis" octahedral molecules, with a same-element pair in a 90 degree angle:
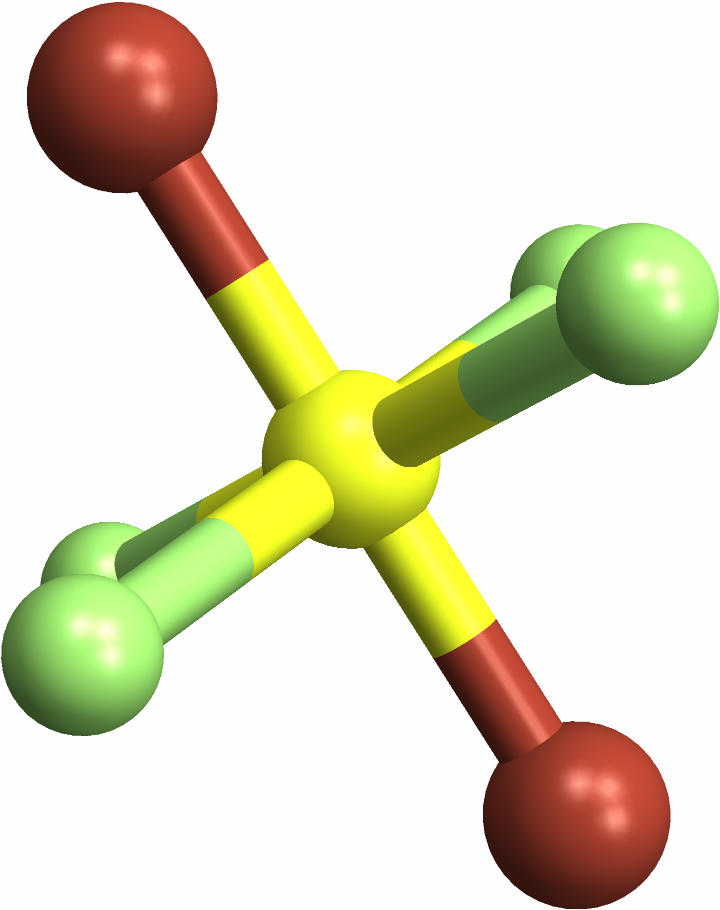
Identifying "trans" octahedral molecules, with a same-element pair opposite each other:
Identifying "fac" octahedral molecules, with three same-element atoms forming a face:
Identifying "mer" octahedral molecules, with three same-element atoms and the central atom forming a plane:
Passing molecules that can't be labeled — that is, molecules that aren't "cis", "trans", "fac", or "mer" — gives you Missing["NotApplicable"]:
The same is true for octahedral molecules:
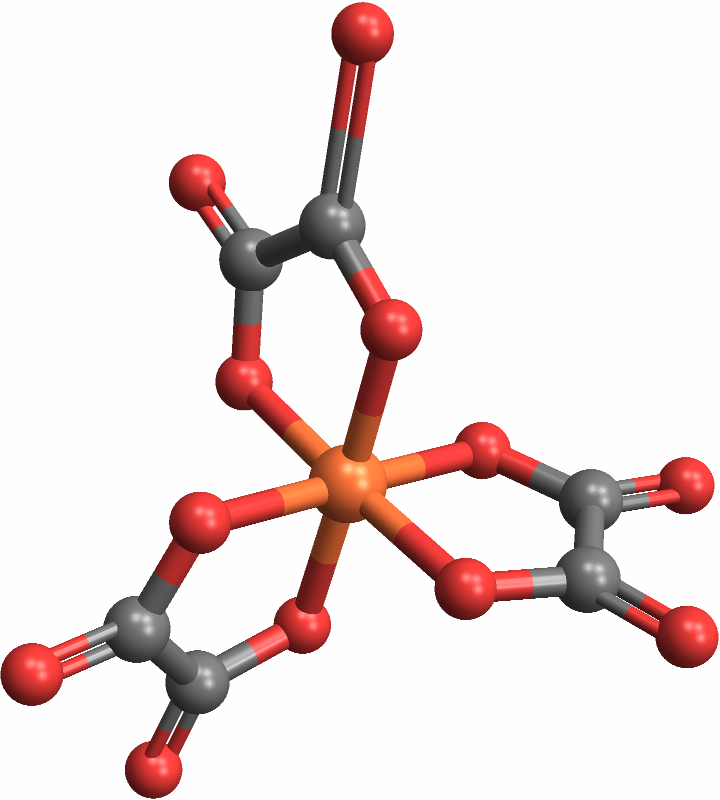
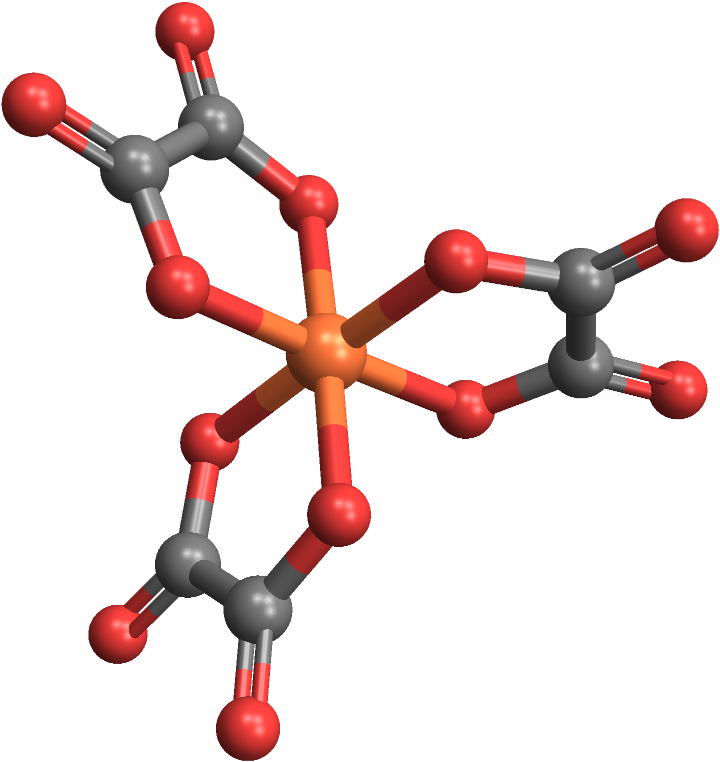
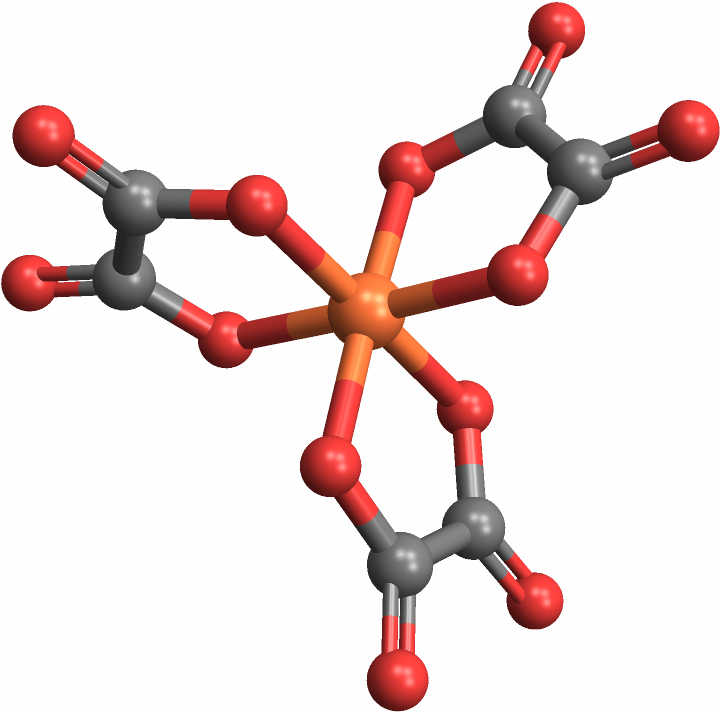
CoordinationMoleculeStereochemistry can handle molecules with polyatomic ligands — central adjacent groups of atoms, rather than singular atoms — as well:
Delta molecules — right-handed molecules, so-called because of the direction in which they turn plane-polarized light — are classified as such:
Lambda molecules — left-handed, named similarly for how they turn light — are classified as such:
Molecules that are neither lambda nor delta isotopes are labeled Missing["NotApplicable"]:
Options (4)
LabelPseudoSymmetric (4)
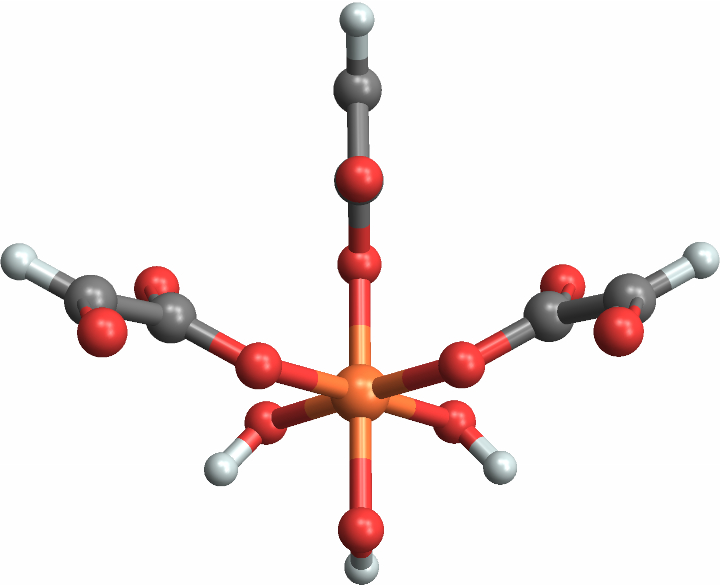
Pseudo-symmetric molecules — molecules whose central-adjacent atoms taken alone can be identified, but not the molecule as a whole — are usually labeled Missing["NotApplicable"]:
Set "LabelPseudoSymmetric" to True to label pseudo-symmetric octahedral molecules:
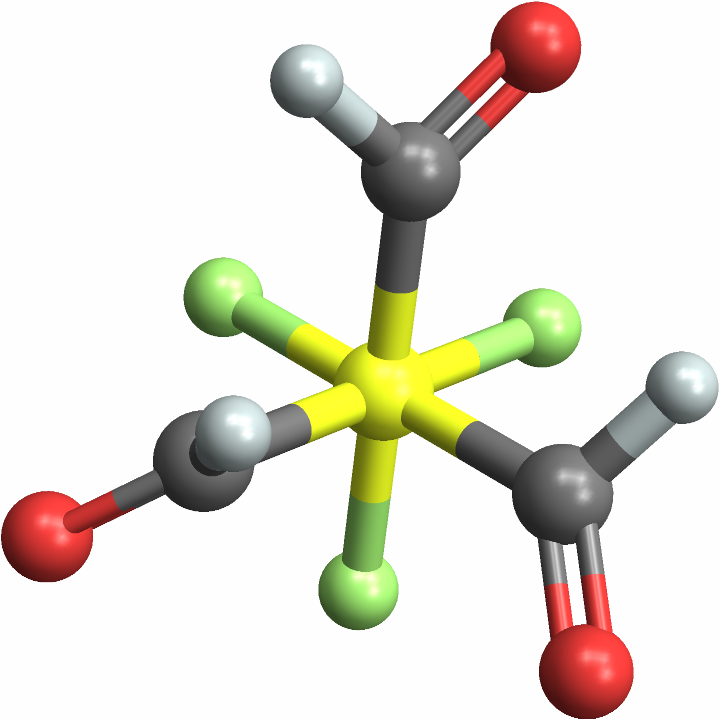
The same is true of optical isomers — pseudo-symmetric isomers are usually labeled Missing["NotApplicable"]:
Set "LabelPseudoSymmetric" to True to label pseudo-symmetric optical isomers:
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/c38aff0e-381f-4703-9623-f90dd81242f2"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/2cbad8c08e143c9b.png)

![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/8b7226dd-db3d-4984-9998-6d04cd148274"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/7acd0bbbcb62de4c.png)
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/c438091e-eea6-41c9-8a16-56642a8351cb"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/7c569797a6d08206.png)
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/abae2437-898a-40a6-a44e-27f05ef1ed67"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/5457fa80a0f9e6a8.png)
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/8a817c13-52eb-4c2e-95a7-db0b2d8d760b"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/57631c1718e94eb6.png)
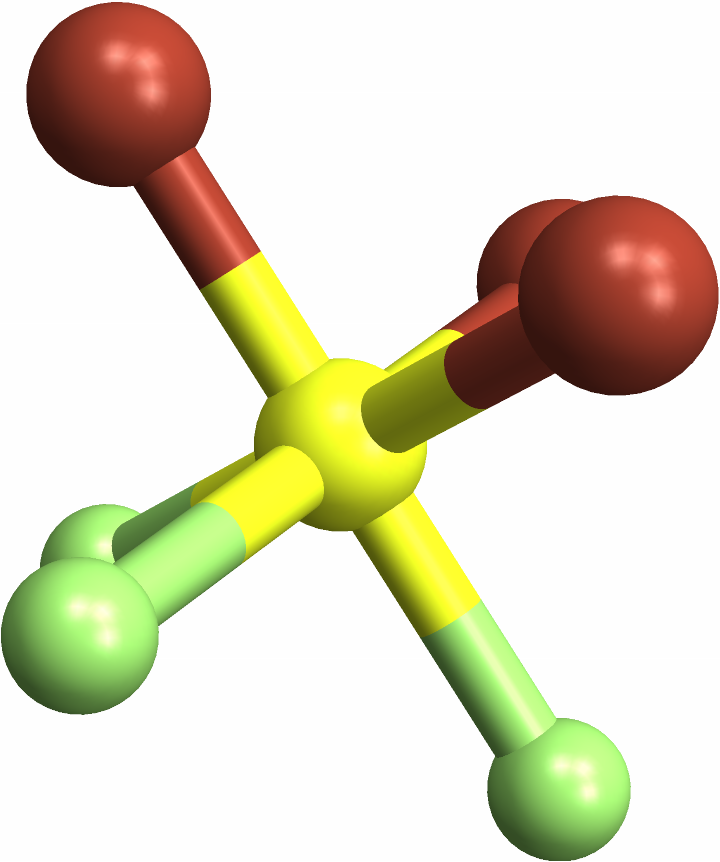
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/a87c0f22-56bb-4b36-b4bc-b71f54d255d2"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/52b9d5023ab745b7.png)
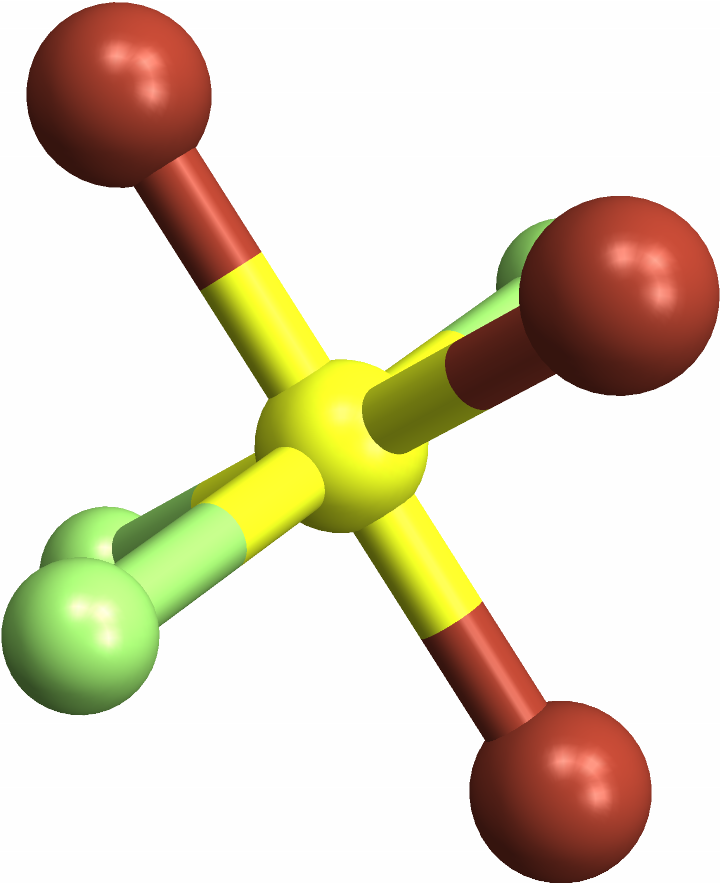
![missingSqPlanar = Molecule[{"F", "Xe", "F", "F", "F"}, {
Bond[{1, 2}, "Single"],
Bond[{2, 3}, "Single"],
Bond[{2, 4}, "Single"],
Bond[{2, 5}, "Single"]}, {AtomCoordinates -> QuantityArray[
StructuredArray`StructuredData[{5, 3}, {{{-1.9203440781716368`, -0.00020447666174505663`, 0.2491154580653996}, {0.000020308462388228532`, 0.00003493564780035485, 0.24881110716492194`}, {
0.0003201650326992592, -1.3578246300191237`, 1.606708105251621}, {1.9200783914456754`, 0.0002795748233125339, 0.24920407308871928`}, {-0.0002795403776389982, 1.3578594957792525`, -1.1090508844212998`}}, "Angstroms", {{
1}, {2}}}]]}];
MoleculePlot3D[missingSqPlanar]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/14cff7ab3937eadc.png)
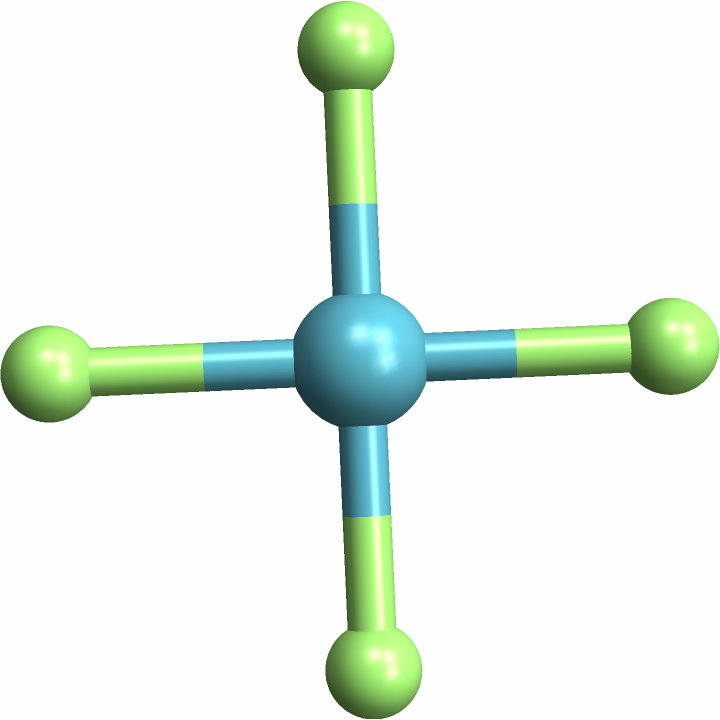
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/50224f22-bd3f-4254-bc19-9b3b465252f3"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/0da17236ed1e3373.png)
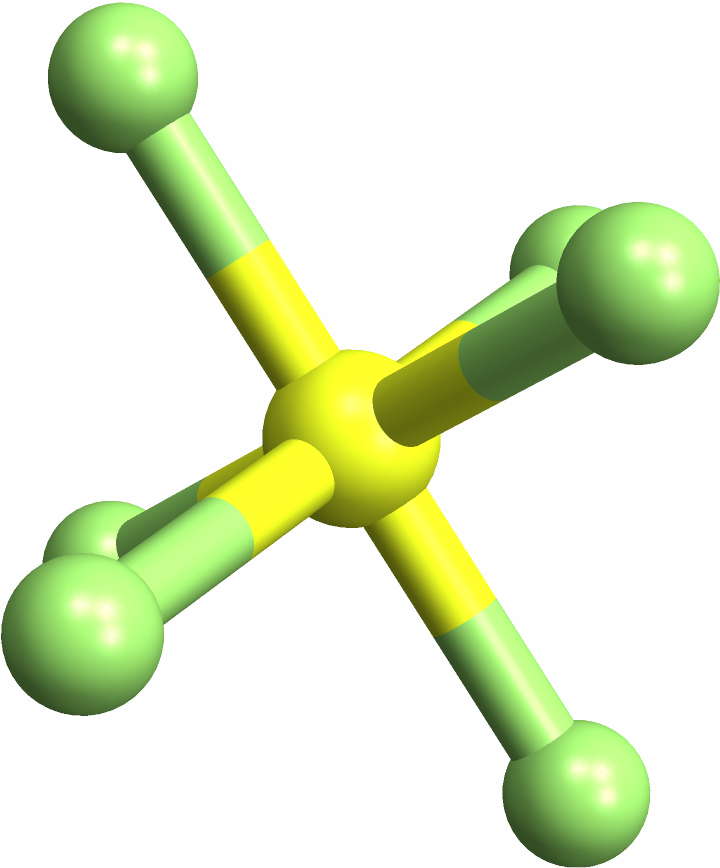
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/e9d2b5c8-1d1e-455e-9e5a-086d488e13b5"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/3f2c5168fcda8301.png)

![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/ba32fc26-f4c6-476d-ad69-0dc91d69d8d4"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/42af680493265a7e.png)
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/564ad1e8-dcce-4956-afeb-3a0bb9c407dd"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/59c325e2a684d2c2.png)
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/c32e0b5d-b550-47f9-9c43-8602bd9f1a14"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/5d4055235a4b1ace.png)
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/9be9246c-9fe3-4442-9b3a-0c6cae581e5c"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/36645166d208475b.png)
![(* Evaluate this cell to get the example input *) CloudGet["https://www.wolframcloud.com/obj/dbba327a-4e88-4a81-ac8a-39292bec7254"]](https://www.wolframcloud.com/obj/resourcesystem/images/e69/e696ed46-b8e3-4ff7-8ed2-907175dc20bf/305f045f1daa35a7.png)