Gas Particles' Velocity
The thermal velocity or thermal speed is a typical velocity of the thermal motion of particles that make up a gas.
The root mean square velocity is proportional to the square root of the Boltzmann constant times the temperature divided by the mass.
Formula
![Copy to Clipboard QuantityVariable[Subscript["v", "rms"], "Speed"] == Sqrt[3]*Sqrt[(Quantity[1, "BoltzmannConstant"]*QuantityVariable["T", "Temperature"])/QuantityVariable["m", "Mass"]]](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/fa3/fa345f2a-626b-4c16-8fcf-485eb43e264f/Webpage/FormulaImage.png)
| symbol | description | physical quantity |
|---|---|---|
| vrms | root mean square velocity | "Speed" |
| m | mass | "Mass" |
| T | temperature | "Temperature" |
Forms
Examples
Get the resource:
| In[1]:= |
| Out[1]= |  |
Get the formula:
| In[2]:= |
| Out[2]= | 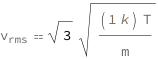 |
Use some values:
| In[3]:= |
| Out[3]= |