pH Using H+ Concentration
pH, the potential of hydrogen, is a numeric scale used to specify the acidity or basicity of an aqueous solution.
pH equals the base 10 logarithm of the H+ concentration.
Formula
![Copy to Clipboard QuantityVariable["pH", "Unitless"] == -(Log[Quantity[1, "Liters"/"Moles"]*QuantityVariable[Row[{"[", Superscript["H", "+"], "]"}], "Molarity"]]/Log[10])](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/cd3/cd3fde58-f0ae-423a-b514-88ce2d0ad49a/Webpage/FormulaImage.png)
| symbol | description | physical quantity |
|---|---|---|
| pH | pH | "Unitless" |
| [H+] | hydrogen-ion concentration | "Molarity" |
Forms
Examples
Get the resource:
| In[1]:= |
| Out[1]= |  |
Get the formula:
| In[2]:= |
| Out[2]= | 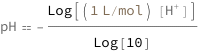 |
Use some values:
| In[3]:= |
| Out[3]= |