Charles's Law
Charles's law states that when the pressure on a sample of a dry gas is held constant, the Kelvin temperature and the volume will be directly related.
Charles's law says that the initial volume divided by the initial temperature equals the final volume divided by the final temperature.
Formula
![Copy to Clipboard QuantityVariable[Subscript["V", "1"], "Volume"]/QuantityVariable[Subscript["T", "1"], "Temperature"] == QuantityVariable[Subscript["V", "2"], "Volume"]/QuantityVariable[Subscript["T", "2"], "Temperature"]](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/bd0/bd0f59fd-511c-4b93-b166-191eb1814ecb/Webpage/FormulaImage.png)
Forms
Examples
Get the resource:
| In[1]:= |
| Out[1]= |  |
Get the formula:
| In[2]:= |
| Out[2]= |
Use some values:
| In[3]:= |
| Out[3]= | 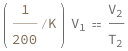 |