Isothermal Process
An isothermal process is a change of a system in which the temperature remains constant.
The final pressure times the final volume equals the initial pressure times the initial volume. The initial and final temperatures are equal. The work done on the system equals the product of the initial pressure, the intial volume and the natural logarithm of the ratio of the initial volume to the final volume. The heat transferred to the system equals the product of the initial pressure, the intial volume and the natural logarithm of the ratio of the final volume to the initial volume. The entropy change equals the product of the initial pressure, the intial volume, the reciprocal of the tmperature and the natural logarithm of the ratio of the final volume to the initial volume.
Examples
Get the resource:
| Out[1]= |  |
Get the formula:
| Out[2]= | 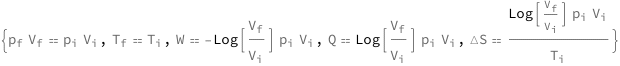 |
Use some values:
| Out[3]= | 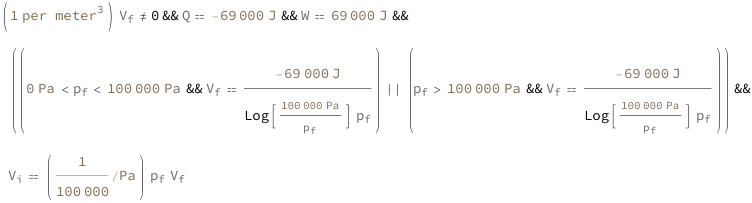 |
External Links
Publisher Information
![Copy to Clipboard {QuantityVariable[Subscript["p", "f"], "Pressure"]*QuantityVariable[Subscript["V", "f"], "Volume"] == QuantityVariable[Subscript["p", "i"], "Pressure"]*QuantityVariable[Subscript["V", "i"], "Volume"], QuantityVariable[Subscript["T", "f"], "Temperature"] == QuantityVariable[Subscript["T", "i"], "Temperature"], QuantityVariable["W", "Work"] == -(Log[QuantityVariable[Subscript["V", "f"], "Volume"]/QuantityVariable[Subscript["V", "i"], "Volume"]]*QuantityVariable[Subscript["p", "i"], "Pressure"]*QuantityVariable[Subscript["V", "i"], "Volume"]), QuantityVariable["Q", "Heat"] == Log[QuantityVariable[Subscript["V", "f"], "Volume"]/QuantityVariable[Subscript["V", "i"], "Volume"]]*QuantityVariable[Subscript["p", "i"], "Pressure"]*QuantityVariable[Subscript["V", "i"], "Volume"], QuantityVariable["ΔS", "Entropy"] == (Log[QuantityVariable[Subscript["V", "f"], "Volume"]/QuantityVariable[Subscript["V", "i"], "Volume"]]*QuantityVariable[Subscript["p", "i"], "Pressure"]*QuantityVariable[Subscript["V", "i"], "Volume"])/QuantityVariable[Subscript["T", "i"], "Temperature"]}](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/b47/b4764fe7-8444-4256-9323-bf52154372b7/Webpage/FormulaImage.png)