Heat Capacity of an Ideal Gas by Amount
Heat capacity or thermal capacity of an ideal gas is a measurable physical quantity equal to the ratio of the heat added (or removed) to the resulting temperature change. An ideal gas is a theoretical gas composed of many randomly moving point particles whose only interaction is perfectly elastic collision.
The isobaric heat capacity of an ideal gas equals the product of a molar gas constant, the amount of gas and the specific heat capacity. The isochoric heat capacity equals the isobaric heat capacity plus the molar gas constant times the amount of gas.
Examples
Get the resource:
| Out[1]= |  |
Get the formula:
| Out[2]= | 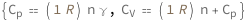 |
Use some values:
| Out[3]= | 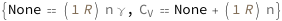 |
External Links
Publisher Information
![Copy to Clipboard {QuantityVariable[Subscript["C", "p"], "HeatCapacity"] == Quantity[1, "MolarGasConstant"]*QuantityVariable["n", "Amount"]*QuantityVariable["γ", "Unitless"], QuantityVariable[Subscript["C", "V"], "HeatCapacity"] == Quantity[1, "MolarGasConstant"]*QuantityVariable["n", "Amount"] + QuantityVariable[Subscript["C", "p"], "HeatCapacity"]}](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/c57/c570c7c7-57c2-40ec-9aff-8bd6dc0dcf8f/Webpage/FormulaImage.png)
