Graham's Law of Diffusion
Graham's law of effusion (sometimes called Graham's law of diffusion) states that the rate of effusion of a gas is inversely proportional to the square root of the mass of its particles.
The ratio of molar flux species A to molar flux species B equals the square root of the ratio of the molecular weight species B to molecular weight species A.
Formula
![Copy to Clipboard QuantityVariable[Subscript["J", "A"], "MolarFlux"] == QuantityVariable[Subscript["J", "B"], "MolarFlux"]*Sqrt[QuantityVariable[Subscript["M", "B"], "MolarMass"]/QuantityVariable[Subscript["M", "A"], "MolarMass"]]](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/636/63627897-f39b-425f-94b5-f4ca6d6f2af3/Webpage/FormulaImage.png)
| symbol | description | physical quantity |
|---|---|---|
| JA | molar flux species A |
"MolarFlux" |
| JB | molar flux species B |
"MolarFlux" |
| MA | molecular weight species A |
"MolarMass" |
| MB | molecular weight species B |
"MolarMass" |
Forms
Examples
Get the resource:
| In[1]:= |
| Out[1]= |  |
Get the formula:
| In[2]:= |
| Out[2]= | 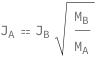 |
Use some values:
| In[3]:= |
| Out[3]= | 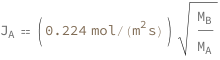 |