De Broglie Wavelength by Kinetic Energy
The de Broglie wavelength is the wavelength associated with a massive particle in motion according to quantum mechanics.
The de Broglie wavelength equals the Planck constant divided by the square root of twice the product of the particle's kinetic energy and mass.
Formula
![Copy to Clipboard QuantityVariable["λ", "Wavelength"] == Quantity[1/Sqrt[2], "PlanckConstant"]/Sqrt[QuantityVariable["K", "Energy"]*QuantityVariable["m", "Mass"]]](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/967/967a17c5-ea7a-4686-a07c-0b1680085f05/Webpage/FormulaImage.png)
| symbol | description | physical quantity |
|---|---|---|
| λ | wavelength | "Wavelength" |
| K | kinetic energy | "Energy" |
| m | mass | "Mass" |
Forms
Examples
Get the resource:
| In[1]:= |
| Out[1]= |  |
Get the formula:
| In[2]:= |
| Out[2]= | 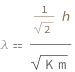 |
Use some values:
| In[3]:= |
| Out[3]= |