pKa Using Concentrations
An acid dissociation constant is a quantitative measure of the strength of an acid in solution. pKa refers to the logarithm of the acid dissociation constant.
The log acidity constant equals the negative of the base 10 logarithm of the product of the conjugate base concentration and the hydrogen ion concentration divided by the acid concentration.
Formula
![Copy to Clipboard QuantityVariable[Subscript["pK", "a"], "Unitless"] == -(Log[(Quantity[1, "Liters"/"Moles"]*QuantityVariable[Row[{"[", Superscript["A", "-"], "]"}], "Molarity"]*QuantityVariable[Row[{"[", Superscript["H", "+"], "]"}], "Molarity"])/QuantityVariable["[HA]", "Molarity"]]/Log[10])](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/65a/65a923de-e14b-464f-9e94-3d3876752a16/Webpage/FormulaImage.png)
| symbol | description | physical quantity |
|---|---|---|
| pKa | log acidity constant | "Unitless" |
| [HA] | acid concentration | "Molarity" |
| [A-] | conjugate base concentration | "Molarity" |
| [H+] | hydrogen ion concentration | "Molarity" |
Forms
Examples
Get the resource:
| In[1]:= |
| Out[1]= |  |
Get the formula:
| In[2]:= |
| Out[2]= | 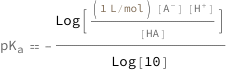 |
Use some values:
| In[3]:= | ![FormulaData[
ResourceObject[
"pKa Using Concentrations"], {QuantityVariable[
Row[{"[", Row[{"H", "+"}], "]"}],"Molarity"] ->
Quantity[0.00042`, ("Moles")/("Liters")], QuantityVariable[
\!\(\*SubscriptBox[\("pK"\), \("a"\)]\),"Unitless"] -> 3.85`,
QuantityVariable[Row[{"[", Row[{"A", "-"}], "]"}],"Molarity"] ->
Quantity[0.25`, ("Moles")/("Liters")]}]](images/65a/65a923de-e14b-464f-9e94-3d3876752a16-io-3-i.en.gif) |
| Out[3]= |