Avogadro's Law
Avogadro's law states that equal volumes of all gases, at the same temperature and pressure, have the same number of molecules. For a given mass of an ideal gas, the volume and amount (moles) of the gas are directly proportional if the temperature and pressure are constant.
The initial volume of an ideal gas divided by the initial amount of the ideal gas equals the final volume divided by the number of the final amount of the ideal gas.
Examples
Get the resource:
| Out[1]= |  |
Get the formula:
| Out[2]= | 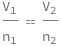 |
Use some values:
| Out[3]= | 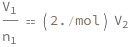 |
External Links
Publisher Information
![Copy to Clipboard QuantityVariable[Subscript["V", "1"], "Volume"]/QuantityVariable[Subscript["n", "1"], "Amount"] == QuantityVariable[Subscript["V", "2"], "Volume"]/QuantityVariable[Subscript["n", "2"], "Amount"]](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/64b/64b38634-5593-4661-ab52-ee5ee3429bf7/Webpage/FormulaImage.png)