Redlich–Kwong Equation of State
The Redlich–Kwong equation of state is an empirical, algebraic equation that relates temperature, pressure and volume of gases.
The pressure generally increases directly with temperature and Redlich-Kwong constant a. Increasing values of Redlich-Kwong constant b and volume tend to smaller values for pressure.
Formula
![Copy to Clipboard QuantityVariable["P", "Pressure"] == (Quantity[1, "MolarGasConstant"]*QuantityVariable["T", "Temperature"])/(-QuantityVariable["b", "RedlichKwongConstantB"] + QuantityVariable["V", "MolarVolume"]) - QuantityVariable["a", "RedlichKwongConstantA"]/(Sqrt[QuantityVariable["T", "Temperature"]]*QuantityVariable["V", "MolarVolume"]*(QuantityVariable["b", "RedlichKwongConstantB"] + QuantityVariable["V", "MolarVolume"]))](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/4df/4df75e4d-ad33-4d88-8d25-a671d23415cf/Webpage/FormulaImage.png)
| symbol | description | physical quantity |
|---|---|---|
| P | pressure | "Pressure" |
| T | temperature | "Temperature" |
| b | Redlich–Kwong constant b | "RedlichKwongConstantB" |
| V | molar volume | "MolarVolume" |
| a | Redlich–Kwong constant a | "RedlichKwongConstantA" |
Forms
Examples
Get the resource:
| In[1]:= |
| Out[1]= |  |
Get the formula:
| In[2]:= |
| Out[2]= | 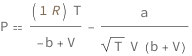 |
Use some values:
| In[3]:= | ![FormulaData[
ResourceObject[
"Redlich\[Dash]Kwong Equation of State"], {QuantityVariable[
"P","Pressure"] -> Quantity[1, "Megapascals"],
QuantityVariable["V","MolarVolume"] ->
Quantity[0.0245`, ("Liters")/("Moles")],
QuantityVariable["T","Temperature"] -> Quantity[298.15`, "Kelvins"],
QuantityVariable["a","RedlichKwongConstantA"] ->
Quantity[0.336`, (Sqrt["Kelvins"] ("Meters")^6 "Pascals")/(
"Moles")^2]}]](images/4df/4df75e4d-ad33-4d88-8d25-a671d23415cf-io-3-i.en.gif) |
| Out[3]= |