Dilution Equation
Dilution is a reduction in the pH of a chemical (gas, vapor, solution). It is the process of decreasing the concentration of a solute in solution, usually simply by mixing with more solvent. To dilute a solution means to add more solvent without the addition of more solute.
The initial concentration times the intial volume equals the final concentration times the final volume.
Examples
Get the resource:
| Out[1]= |  |
Get the formula:
| Out[2]= | 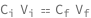 |
Use some values:
| Out[3]= | 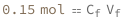 |
External Links
Publisher Information
![Copy to Clipboard QuantityVariable[Subscript["C", "i"], "Molarity"]*QuantityVariable[Subscript["V", "i"], "Volume"] == QuantityVariable[Subscript["C", "f"], "Molarity"]*QuantityVariable[Subscript["V", "f"], "Volume"]](https://www.wolframcloud.com/objects/resourcesystem/marketplacestorage/resources/3cd/3cd992b8-0ac0-40d1-b155-b39f1809f79d/Webpage/FormulaImage.png)
